The website has the complete lesson note for all the subjects in secondary school but this piece showcases the SS1 Chemistry Lesson Note on Laws of Chemical Combination. You can use the website search button to filter out the subject of interest to you.
CLICK HERE to download the complete Document: DOWNLOAD HERE
TOPIC: LAWS OF CHEMICAL COMBINATION
CONTENT:
- Law of conservation of matters
- Law of constant composition
- Law of multiple proportions.
PERIOD 1: CHEMICAL LAWS OF COMBINATIONS
There are four laws of chemical combination which describe the general features of a chemical change.
- Law of conservation of mass: This law was established by Lavoisier, a French chemist. The law of conservation of mass states that matter is neither created nor destroyed during chemical reaction, but changes from one form to another.
Experiment to verify the law of conservation of matter (mass)
Theory:
The equation of the chemical reaction chosen for study is as follows;
Silver nitrate + sodium chloride Silver chloride + Sodium trioxonitrate(v)
(White precipitate)
Method:
- Put some sodium chloride solution in a conical flask
- Fill a small test tube with silver trioxonitrate (iv) solution of string, suspend it in a conical flask as shown below:
- Insert the stopper and weight the whole apparatus on a balance, note the mass of the whole system.
- Mix the two liquids by pulling the string attached to the bottom end of the small test tube.
- Weigh the whole apparatus again.
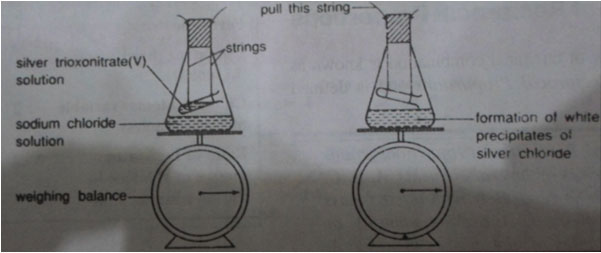
Result: When the two reactants are mixed together, a white precipitate is formed indicating that a chemical reaction has taken place. The new substances formed are known as the products of the chemical reaction. The masses of the system taken before and after the reaction are found to be the same, indicating that the mass of the reactants equals that of the products.
CONCLUSION: Since there is no overall change in mass when the products are formed, we can infer that matter is neither created nor destroyed during the chemical reaction. The law is, hence valid.
Click on the Downloadable Button to get the FULL NOTE


