The website has the complete lesson note for all the subjects in secondary school but this piece showcases the SS2 Chemistry Lesson Note on Water. You can use the website search button to filter out the subject of interest to you.
CLICK HERE to download the complete Document: DOWNLOAD HERE
TOPIC: WATER
CONTENT
- Structure of water, Hardness of water and removal of hardness of water
- Solubility- Basic concept
- Types of Solutions
- Factors affecting solubility and uses of solubility curve.
PERIOD 1: STRUCTURE OF WATER, HARDNESS OF WATER AND REMOVAL OF HARDNESS OF WATER
A molecule of water consists of two atoms of hydrogen and one atom of oxygen chemically combined to form a covalent molecule. A molecule of water has a V-shape. The repulsion between the two lone pairs of electrons on the oxygen atom makes the structure of water molecular to be V- shaped
Structure of water
(a) Bonding
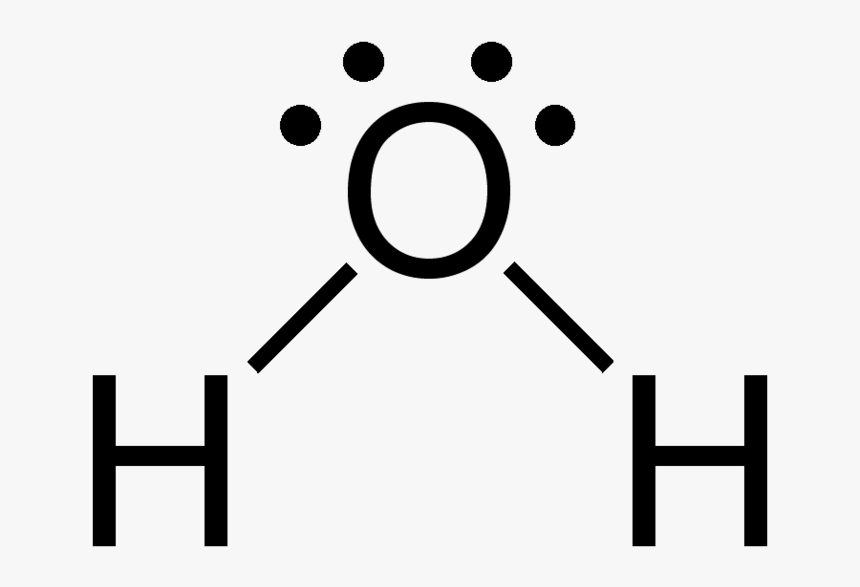
(b) Shape of water molecule
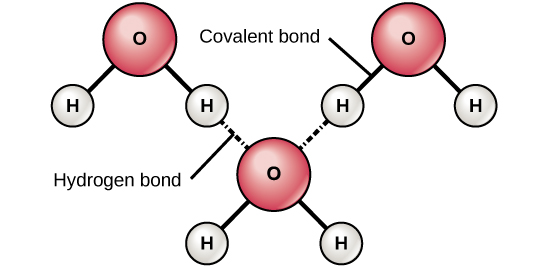
The shared pair of electrons between each hydrogen atom and oxygen is drawn more electronegative, making the oxygen atom to be partially negatively charged and hydrogen atoms to be partially positively charged. This leads to hydrogen bonding between the molecules of water the attendant relatively high melting point, high boiling point and low vitality.
Hydrogen bonding in water molecules
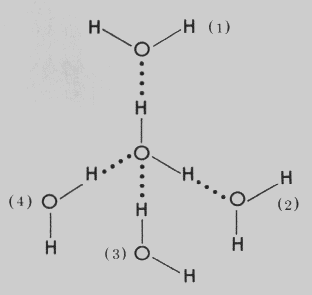
HARDNESS OF WATER
A hard water is water which will not readily form lather with soap. For example, river water, lake water, stream water and sea water. Hardness of water is due to the presence of calcium, magnesium and iron in water. There are two types of hard water: temporary hardness and permanent hardness.
TEMPORARY HARDNESS: This is caused by the presence of dissolved calcium or magnesium hydrogen trioxocarbonate(IV), HCO3– of Ca, Mg or Fe. Temporary hardness is so called because it is easily removed by boiling.
Removal of temporary hardness: Temporary hardness can be removed by:
- Boiling: The calcium hydrogen trioxocarbonate(IV) which causes the temporary hardness is decomposed by heating.
- Addition of calculated amount of slaked lime, Ca(OH)2
Ca(HCO3)2 + Ca(OH)2 → 2CaCO3 + 2H2O
Soluble slightly soluble → insoluble
Effects of temporary hardness:
- Furring of kettle: When a kettle or boiler has been used to boil hard water for some time, the inner surface becomes coated with a white fur-like layer. The layer is due to the gradual deposit of CaCO3 from the decomposition of Ca(HCO3)2
- Stalagmites and stalactites: These are pillars of limestone, CaCO3.
Found in hot caves. They are formed when hard water flow temporary over the top of the cave and drop off water then becomes decomposed by the heat inside the cave, leaving deposits of CaCO3 on the roof and floor of the cave. The deposits that grow downward are termed stalactites and the ones that grow upward are termed stalagmites. Pillars of CaCO3 are formed when both stalactites and stalagmites meet.
PERMANENT HARDNESS: This is caused by the presence of dissolved calcium and magnesium ions in form of soluble tetraoxosulphate(VI) and chlorides and certainly cannot be removed by boiling. When water containing any of these substances is evaporated, a white solid deposit of calcium or magnesium tetraoxosulphate(VI) and/ or calcium trioxocarbonate(IV) is left behind. Calcium trioxocarbonate(IV) causes the ‘furring’ in kettles that occur in hard water. This furring may be removed by the addition of a dilute acid:
2H+ + CO32-(aq) → CO2(g) + H2O(l)
Click on the Downloadable Button to get the FULL NOTE



