The website has the complete lesson note for all the subjects in secondary school but this piece showcases the SS2 Chemistry Lesson Note on Alkanols. You can use the website search button to filter out the subject of interest to you.
CLICK HERE to download the complete Document: DOWNLOAD HERE
TOPIC: ALKANOLS
CONTENT:
- CLASSES AND TYPES OF ALKANOLS
- LABORATORY AND INDUSTRIAL PRODUCTION OF ALKANOLS,
- PHYSICAL AND CHEMICAL PROPERTIES OF ALKANOLS
- LABORATORY TEST AND USES OF ALKANOLS.
PERIOD 1: CLASSES AND TYPES OF ALKANOLS
CLASSES OF ALKANOLS
There are three classes of alkanols – primary, secondary and tertiary.
- Primary ( ) alkanols: These have only one alkyls group attached to the carbon atom that carries the hydroxyl group e.g. C2H5OH, C4H9OH etc. Example: C2H5OH,
- Secondary ( ) alkanols: These have two alkyls attached to the carbon that carries the hydroxyl group e.g. Example: CH3 CH2 CH (OH) CH3 (Botan – 2- ol)
- Tertiary ( ) Alkanols: These have three alkyl group attached to the carbon atom that carries the hydroxyl group. Example:
C (CH3)3OH (2-methylpropan-2-ol).
TYPES OF ALKANOLS
- Trihydric alkanols: These are alkanols with three (3) OH groups per molecule e.g. propane-1, 2, 3-triol
- Dihydric alkanols: These contain 2 OH groups per molecule e.g. ethane-1, 2-diol
- Monohydric alkanols: These contain only one (1) OH group per molecule e.g. ethanol, C2H5OH, etc.
They have the general molecular formula CnH2n+1OH. This is sometimes written as ROH. Where R stands for an alkyl group
Examples of the first five members of the group are:
| Chemical Formula | Name | Structural Formula |
|
CH3OH |
Methanol |
|
|
C2H5OH |
Ethanol |
|
|
C3H7OH |
Propanol |
|
|
C4H9OH |
Butanol |
|
|
C5H11OH |
Pentanol | 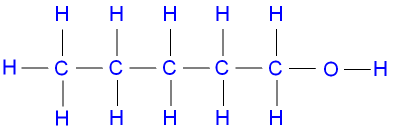 |
Click on the Downloadable Button to get the FULL NOTE




