The website has the complete lesson note for all the subjects in secondary school but this piece showcases the SS1 Chemistry Lesson Note on Hydrocarbon and its Main Classes. You can use the website search button to filter out the subject of interest to you.
CLICK HERE to download the complete Document: DOWNLOAD HERE
TOPIC: CARBON AND ITS COMPOUNDS
CONTENTS:
- HYDROCARBON AND ITS MAIN CLASSES
- CRUDE OIL AND NATURAL GAS- FRACTIONAL DISTILLATION OF CRUDE OIL AND USES OF PETROLEUM FRACTIONS
- CRACKING OF PETROLEUM FRACTIONS, REFORMING, OCTANE NUMBER AND KNOCKING
- IMPORTANCE OF CRUDE OIL AND PETROCHEMICALS
PERIOD 1: HYDROCARBON AND ITS MAIN CLASSES
Hydrocarbons, as the name implies, are compounds of only two elements; hydrogen and carbon. There are many such organic compounds.
Hydrocarbons are usually classified into two main groups namely, saturated and unsaturated hydrocarbons.
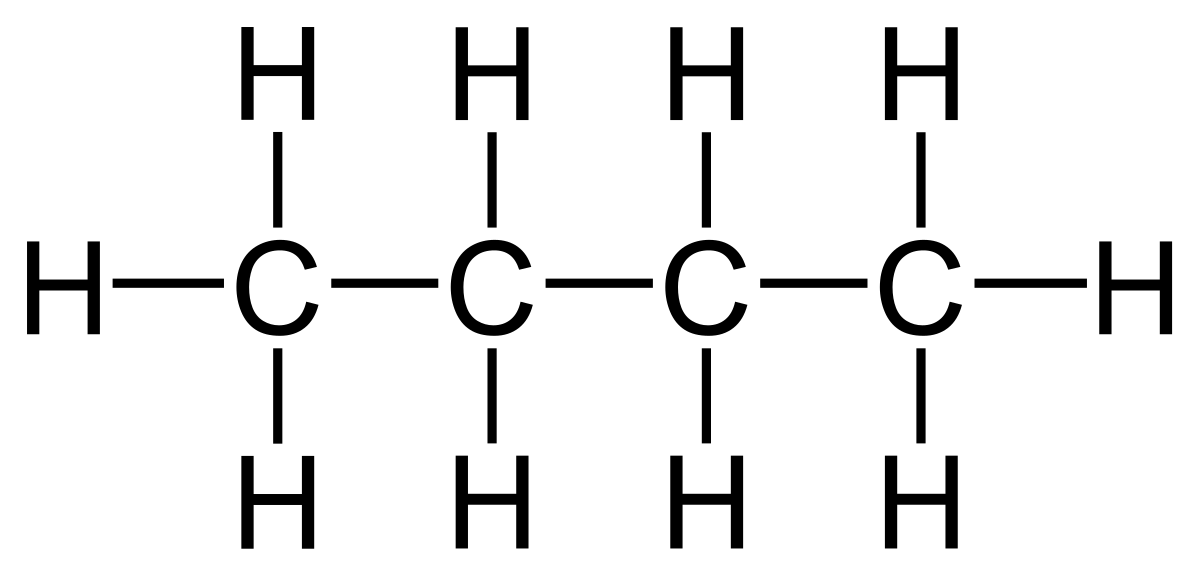
- Saturated hydrocarbons (Alkanes). The alkanes have the general molecular formula CnH2n+2. . Members of this group include Methane, CH4, ethane, C2H6, propane, C3H8, butane, C4H10. The structure of butane is given below.
- Unsaturated hydrocarbons (Alkenes and alkynes). The alkenes have the general formula is CnH2n. Members of this group include ethene, C2H4 propene, C3H6 butene, C4H8 etc
Hydrocarbons can be classified into:
- Aliphatic compounds
- Aromatic compounds
Aliphatic compounds are classified into two sub-classes: 1. Acyclic compounds 2. Cyclic compounds
In acyclic compounds the molecules are made up of straight chain carbon atoms or branch chain e.g.
Butane (a straight chain compound)
2 methyl butane (a branched chain compound)

In cyclic compounds, the end carbon atoms of an open aliphatic chain can also join together to form a closed system or ring e.g.
Cyclopropane
AROMATIC COMPOUNDS
Aromatic compounds are Benzene or derivative of benzene (that is compounds whose structures are based on the structure of benzene)
Due to resonance exhibit by the structures of benzene, i.e.
- (ii)
The structure of benzene would be (i) or (ii)
Conventionally the structure of benzene is now a hexagon with a ring within it.

Examples of other derivatives of benzene:
Click on the Downloadable Button to get the FULL NOTE



![A hydrocarbon contained 14.3% hydrogen. The empirical formula for the hydrocarbon is [H= 1.0, C= 12.0] A hydrocarbon contained 14.3% hydrogen. The empirical formula for the hydrocarbon is [H= 1.0, C= 12.0]](https://erudites.ng/wp-content/plugins/contextual-related-posts/default.png)
