The website has the complete lesson note for all the subjects in secondary school but this piece showcases the SS1 Chemistry Lesson Note on Graphite. You can use the website search button to filter out the subject of interest to you.
CLICK HERE to download the complete Document: DOWNLOAD HERE
GRAPHITE
Structure and Bonding
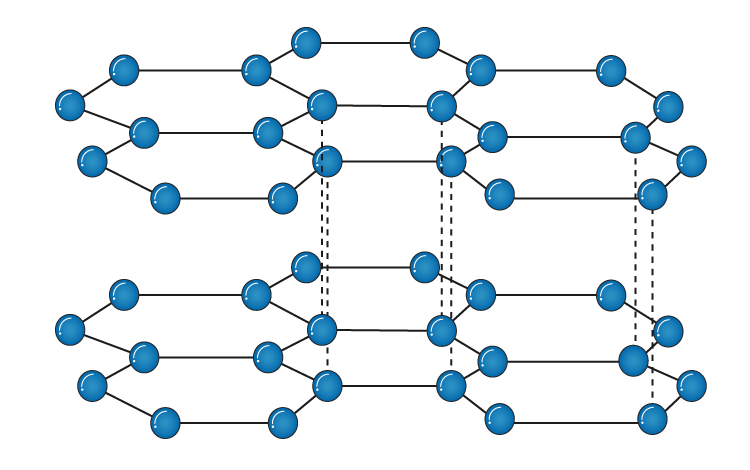
In graphite, each carbon atom is covalently bonded to three other atoms to produce an infinite two-dimensional flat hexagonal layer structure, which is strong and hard. See the diagram above. The flat hexagonal layers in graphite are held together by the weak van der Waals attractive forces, which allow movement of the planes parallel to each other, and make the graphite to be soft and slippery. The fourth electron in the valence shell of each carbon atom in graphite is mobile, because it is not used in bonding, and account for its electrical conductivity.
EVALUATION
- Define the term allotropy.
- What is the structure of? (a) diamond (b) graphite.
- Give the reason why diamond is hard, while graphite is soft.
PERIOD 2: PROPERTIES, DIFFERENCE BETWEEN DIAMON AND GRAPHITE
Physical properties of graphite
- Graphite forms soft, black and opaque hexagonal crystals, which are greasy to feel. The softness is due to the ability of the adjacent layers to slide over one another.
- It is hard. Its density is 2.3gcm-3, and melts at about 35000
- It is good conductor of heat and electricity-due to the presence of a mobile electron per carbon atom.
- It is soluble in any solvent. Graphite is an example of a non –metallic conductor.it is a metalloid.
USES OF GRAPHITE
- Graphite is used as a lubricant, because of its flat hexagonal layer which can slide over one another.
- Used as inert electrodes during electrolysis and for brushes of electric motors been a good conductor of electric current.
- When mixed with clay, graphite forms lead, which is used in making lead pencils. The hardness of a pencil depends on the amount of clay in the mixture. Soft pencils contain more of graphite, while hard pencils contain more of clay.
- Used in making crucible, because of its high melting point.
- Used in nuclear reactors; being soft and with a high melting point.
DIFFERENCE BETWEEN DIAMOND AND GRAPHITE
| DIAMOND PROPERTIES | GRAPHITE PROPERTIES |
| 1. Diamond is a transparent solid that sparkles when cut and polished | Graphite is an opaque solid, with a metallic luster |
| 2. It is octahedral in shape | It is hexagonal in shape |
| 3. Its density is 3.5 | Its density is 2.3 |
| 4. It is a poor conductor of electricity | It is a good conductor of electricity |
| 5. It is an inert substance but at 9000c, it burns in air to form carbon(iv) oxide and combines with fluorine | It is a more reactive substance burns in air to form carbon (iv) oxide at 7000c, it also reacts with oxidizing agents to form oxides, it also reacts with fluorine and tetraoxosulphate(vi) acid |
| 6. It is the hardest substance known | It is one of the softest minerals known. |
Click on the Downloadable Button to get the FULL NOTE




