The website has the complete lesson note for all the subjects in secondary school but this piece showcases the SS1 Chemistry Lesson Note on Carbon (IV) Oxide (CO2): Preparations; Properties and Uses. You can use the website search button to filter out the subject of interest to you.
CLICK HERE to download the complete Document: DOWNLOAD HERE
TOPIC: CARBON AND ITS COMPOUND
CONTENT:
- CARBON(IV) OXIDE (CO2): PREPARATIONS, PROPERTIES AND USES.
- CARBON(II) OXIDE, CO: PREPARATIONS, PROPERTIES AND USES. SYNTHETIC GASES
- METALLIC TRIOXOCARBONATES; OCCURRENCES, PREPARATION AND USES.
- TEST FOR CARBON ION
PERIOD 1: CARBON(IV) OXIDE: PREPARATION AND USES
FORMATION / PREPARATION OF CARBON(IV) OXIDE (CO2)
In excess air, there is complete combustion; carbon (iv) oxide is produced but in the limited supply of air carbon normally reacts with oxygen to produce carbon(ii) oxide. The equation of reaction is given below for the two reactions.
C(s)+ O2(g) → CO2(g) + heat (sufficient supply of air)
2C(s) + O2(g) → 2CO(g) + heat (limited supply of air)
Other methods of preparation of CO2 are:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
- The decomposition of trioxocarbonates(IV) salts [except those of Sodium and Potassium] or hydrogen trioxcarbonate(IV) salts, by strong heat e.g
CaCO3(s) → CaO(s) + CO2
2NaHCO3(s) → Na2CO3(s) + H2O +CO2(g)
- Action of dilute mineral acid on trioxocarbonate(IV) or hydrogentrioxocarbonate(IV)salt e.g.
Na2CO3(s) + 2HCl(aq) → 2 NaCl(aq) + H2O(l) + CO2(g)
KHCO3(s) + HNO3(aq) → KNO3(aq) + H2O(l) + 2CO2(g)
(iv) The fermentation of glucose by an enzyme (zymase)
C6H12O6(ag) → 2C2H5OH(aq) + 2CO2(g)
LABORATORY PREPARATION OF CO2 GAS
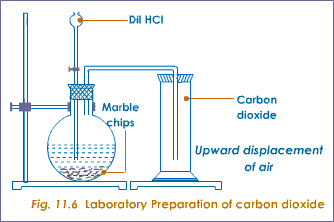
The equation for the reaction is given as shown below
CaCO3(s) + HCl(aq) → CaCl2(aq) + H2O + CO2(g)
Pure and dry CO2 is obtained by passing the gas through a solution of potassium hydrogentrioxocarbonate (IV) [KHCO3] to remove the acid impurities, hydrogen chloride and then, through conc. (or fused CaCl2 to dry the gas. It is then collected by downwards delivery, being denser than air as in the diagram above.
PHYSICAL PROPERTIES OF CO2(g)
Click on the Downloadable Button to get the FULL NOTE




