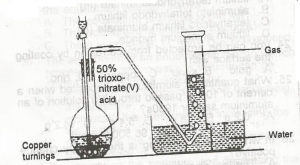
A. NO
B. NO2
C. N2O
D. N2O4
Correct Answer:
Option A – NO
Action of dilute (50% of HNO3) will give 8HNO3(l) + 3Cu(s) → 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l) Whereas
Action of Conc HNO3 will give 4HNO3(l) + Cu(s) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l) The copper nitrate salt that forms is a deep blue colour. The nitrogen dioxide is a maroon vapour.




