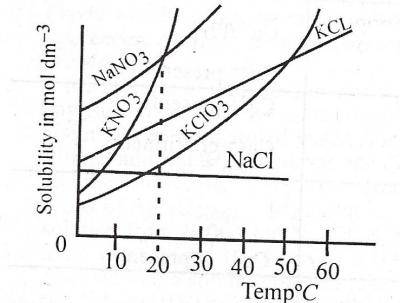
A. 1.5 mol dm−3
B. 1.5 mol dm−3
C. 2.0 mol dm−3
D. 5.0 mol dm−3
Correct Answer:
Option A = 1.5 mol dm−3
Explanation
V= 50cm3
Mass= 5.05g
Relative molecular mass of KNO3 = (39+14+(3*16)) = 101
Convert 50cm3 to dm3 which is
1000cm³ = 1dm3
50cm³ = 50*1/1000
= 0.05dm3
Moles = mass/ molar mass
= 5.05/101 =0.05mole
Solubility= mole/volume
Solubility=0.05mol/0.05dm3
Solubility=1.0mol/dm_3



![A quantity of electricity liberates 3.6g of Silver from its salt. What mass of aluminium will be liberated from its salt by the same quantity of electricity? [Al = 27, Ag = 108]. past-questions-and-answers](https://erudites.ng/wp-content/uploads/2020/06/past-questions-and-answers-150x150.jpg)